You have a cup of water suspended in a pot of boiling water.
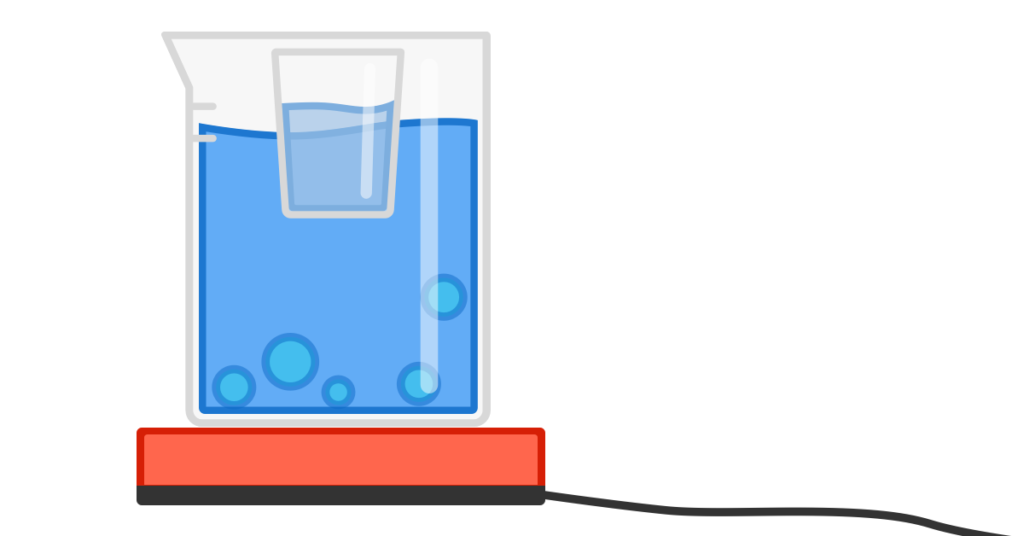
Will the water in the cup ever boil? (We will assume that the pot doesn’t boil dry.)
The first new player to comment on the website with the correct answer (and an explanation) wins a free drink at their next iQ Trivia show.
Assuming there is water in the big pot and the ambient temperature is below 100 C (i.e. you haven’t put the whole lot inside an oven), the water in the cup will not boil. The big pot’s water will not exceed 100 C, neither will the vapour coming off it. Because the cup provides some insulation, even if it is metallic, the water in the cup will be slightly under 100 C therefore not boil. For information in takes 540 times as much heat to vaporise a quantity of water at 100 C than to take it from 99 to 100 C. (Latent heat of vaporisation)
No it won’t boil for the reasons you detailed.
(And thanks for the additional explanation on the latent heat of vaporisation.)